mm.X product family
Clinical studies and observations
The clinical performance of the mm.X implants is the most important factor for successful patient care. In all sub-steps of the development, every detail was examined in order to achieve the symbiosis of safety for the patient, tissue compatibility, mechanical function and controlled conversion into intact bone tissue.
In cooperation with leading clinical partners, high-quality studies are carried out and published under scientific standards. As soon as the first results are obtained, they will be shown and published here. If you are interested in working with us, we look forward to hearing from you.
Tissue compatibility
Tissue compatibility
Bioabsorbable implants always have an influence on the surrounding tissue and interact with it. mm.X implant show a very good integration into the surrounding tissue. The material shows an osteoconductive effect and stimulates the bone to rebuild.
The preclinical tests were conducted exclusively in Germany at leading scientific institutions. The evaluation of the tissue samples and results were carried out far beyond the legal requirements.
Tissue compatibility
Tissue compatibility
Bioabsorbable implants always have an influence on the surrounding tissue and interact with it. mm.X implant show a very good integration into the surrounding tissue. The material shows an osteoconductive effect and stimulates the bone to rebuild.
The preclinical tests were conducted exclusively in Germany at leading scientific institutions. The evaluation of the tissue samples and results were carried out far beyond the legal requirements.
Controlled Absorption
Numerous in vitro data demonstrate the effectiveness of material technology in the rate of bioabsorption. Hydrogen formation is directly proportional to the rate of degradation of the material. A controlled degradation achieved by the material technology provides the bone with enough time for a safe healing.
The mm.X platform has been extensively analyzed for a controlled bioabsorption in tissue and bone. In several preclinical experiments, biocompatibility and a controlled bioabsorption was demonstrated.
Controlled Absorption
Numerous in vitro data demonstrate the effectiveness of material technology in the rate of bioabsorption. Hydrogen formation is directly proportional to the rate of degradation of the material. A controlled degradation achieved by the material technology provides the bone with enough time for a safe healing.
The mm.X platform has been extensively analyzed for a controlled bioabsorption in tissue and bone. In several preclinical experiments, biocompatibility and a controlled bioabsorption was demonstrated.
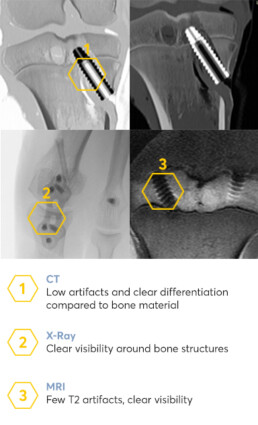
Precise radiological diagnostics
The implants are very well usable in all relevant radiological procedures and allow targeted diagnoses. The implants are clearly visible in X-ray images and it is possible to make a clear statement about fracture healing. Furthermore, they are well characterizable in CT scans. Compared to titanium implants, Medical Magnesium implants have significantly fewer T2 artifacts during MRI. Targeted images from both MRI and CT scans are well suited to track the progress of implant degradation.
Precise radiological diagnostics
The implants are very well usable in all relevant radiological procedures and allow targeted diagnoses. The implants are clearly visible in X-ray images and it is possible to make a clear statement about fracture healing. Furthermore, they are well characterizable in CT scans. Compared to titanium implants, Medical Magnesium implants have significantly fewer T2 artifacts during MRI. Targeted images from both MRI and CT scans are well suited to track the progress of implant degradation.