When developing novel and innovative medical devices, it is usually necessary to actively generate safety and performance data on patients. These data are generated in clinical trials and are required to demonstrate compliance with regulatory requirements. The safety of a medical device depends on product related risks and on the complexity of its application. The more complex the application of a device, the greater the associated risk. While device-specific risks are addressed during design development, application risks are minimized by user training during clinical practice. Because the safety of participants is of highest priority in a clinical trial, investigators should already gain experience with the investigational device and acquire a certain routine in its use prior to enrolment of the first study subject.
Investigator training
The use of magnesium implants does not require any special surgical procedure or method that deviates from the clinical standard. However, the mechanical properties of magnesium alloys differ from those of titanium or stainless steel. Thus, mm.X implants such as the mm.IF interference screw or the mm.CS compression screw demonstrate unique insertion behavior and specific mechanical load capacities. Before treating the first patient in a clinical trial, investigators should already be familiar with the mechanical properties and the correct handling of mm.X implants and instruments. This requires training under the most realistic conditions such as the implantation of mm.X on human specimen. As part of each clinical trial preparation, we therefore arrange investigator meetings with training sessions at the Cadlab Cologne, a top-class training facility for the surgical education of physicians on freshly frozen human specimens. Apart from the training, we use the meetings to discuss the most important points of the studies and offer all study investigators the possibility to interact and to get to know each other.
Insights from mm investigator meetings
The first part of each investigator meeting is used to present the clinical trial to the investigators (see Figure 1, right picture).

Figure 1: Explanation of study activities during the mm.IF investigator meeting at Film Studios Cadlab Cologne.
During this session, investigators can give input and discuss open questions while considering the foreseen measures for data collection, eligibility criteria for study participants, visit plans as well as objectives and endpoints of the study. The session is shared online to allow virtual participation of investigators who cannot attend in place. The investigator meetings are carried out prior to finalization of the study protocol and approval by ethics committees and competent authorities. Thus, it can be ensured that the results of the discussions are adequately considered for the clinical trial.
An introduction into the second session is given by our product manager who presents the available specimen, the surgical procedures, and the mm.X implants that are relevant for the clinical trial. This is followed by mm.X training on human specimen. Always two operation teams can work simultaneously on separate specimens to minimize waiting times. Investigators choose the surgical procedure, the required device configuration and perform the mm.X implantation, similar as done during regular practice and in the clinical study. After each surgery, the results are visualized by X-ray imaging and discussed together. At any time, medical magnesium staff is available for questions, suggestions, and hand-on support for the surgeon (see Figure 2). This offers an optimal environment for investigators to familiarize with the implants, the available configurations and the instruments intended to be used in the clinical trial. Like the first session of the event, also the surgical training session is shared online. This allows all investigators, who cannot attend in place, to participate, to ask questions and to give input any time.
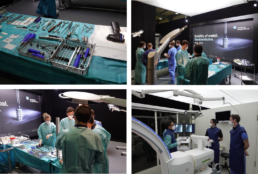
Figure 2: Impressions of the mm.IF study investigator training at Film Studios Catlab Cologne.
In the third and final session of the event, investigators have the possibility to check out the mm.X portfolio independent from the clinical trial (see Figures 2 and 3). What kind of surgery and implants can be applied depends on the human specimen available, e.g., an isolated foot or an entire leg. Interference screws (mm.IF), compression screws (mm.CS) and implants for forefoot surgery (mm.PIP) are available. Usually, investigators use this session to test mm.X implants they have no experience with, and to practice new or unfamiliar surgical procedures to improve personal skills. In all cases, the results of the surgeries are visualized by X-ray imaging and provided to the surgeons afterwards.

Figure 3: Testing session (left) and closing of the mm.PIP investigator meeting (right) at Catlab Cologne.
Conclusion
With the help of study-specific and realistic training events, investigators are adequately trained on mm.X implants, available device configurations and instrument handling prior to trial initiation. This measure ensures the safety of the study participants in the best possible way.
